Organizations using both a RIM Vault and a Clinical Operations Vault on the same domain can use a standard Vault to Vault connection. When configured, the RIM-Clinical Operations connection transfers Product Family, Clinical Study, and Clinical Site records, as well as regulatory data, across Vaults. The connection also and automates the creation, versioning, and updating of CrossLink documents and fields. Streamlining object record and document creation reduces duplicate data and allows Vault documents to have a single source of truth within an organization.
How the RIM-Clinical Operations Connection Works
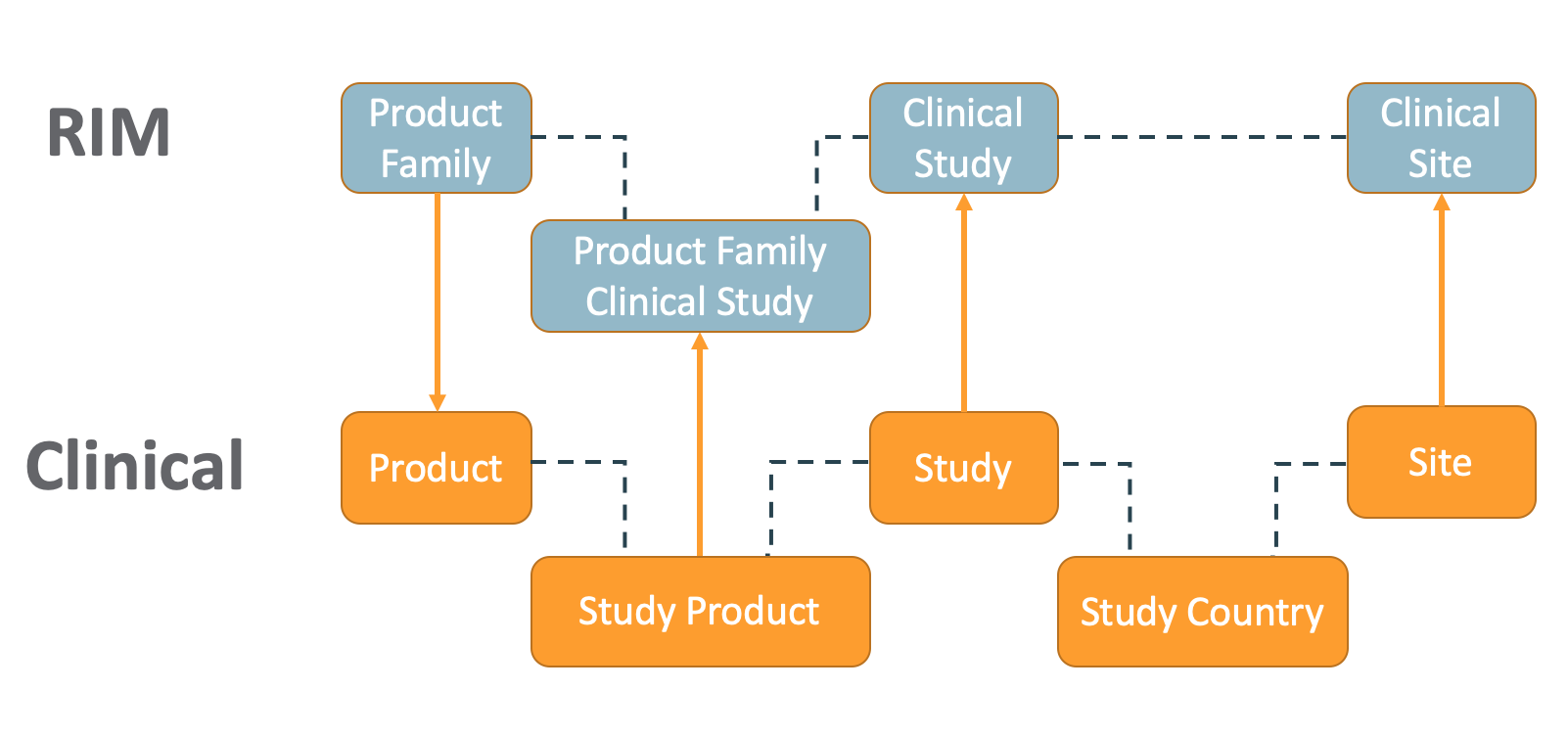
The RIM-Clinical Operations Connection creates new records, updates existing records, and creates CrossLink documents across Vaults in the below situations.
Object Records
- When a user creates or edits a Product Family record in the RIM Vault, Vault creates or updates the Product record in the Clinical Operations Vault.
- When a user creates or edits a Study record in the Clinical Operations Vault, Vault creates or updates the Clinical Study record in the RIM Vault.
- If the Study Type, Study Subtype, or Type of Control fields are populated on the Study record, Vault updates the Clinical Study record with the appropriate mapped fields in RIM.
- When a user creates or edits a Study Product record in the Clinical Operations Vault, Vault creates or updates the Product Family Clinical Study record in RIM.
- When a user creates or edits a Site record in the Clinical Operations Vault, Vault creates or updates a Clinical Site record under the same Clinical Study record in RIM and populates the Site Country field based on Study Country in the Clinical Operations Vault.
- When a user creates or edits a Regulatory Objective or Country Decision Detail record related to a clinical trial approval Application in RIM, Vault updates a corresponding milestone record in Clinical Operations.
CrossLink Documents & Fields
- When a document enters its Steady state in the source Vault, Vault creates or versions a CrossLink document in the target Vault and populates fields. Admins can also configure Vault to transfer document versions to the connected Vault when they reach the source Vault’s Superseded state. An Admin defines CrossLink document behavior in the connection’s configuration. See details about document creation below.
- Study field behavior for CrossLinks created in Clinical Operations from RIM:
- When a RIM source document has a valid Clinical Study field value (according to the Clinical Operations Study), the connection populates it accordingly. If the Study does not exist in Clinical Operations, the connection generates a User Exception Message.
- When a RIM source document has a blank Clinical Study field value, Vault populates the Clinical Operations Study field based on the Active Lead Agent Product associated with the Product Families on the RIM document. In order to leverage this defaulting behavior, Study records in Clinical Operations must use the Lead Agent Study Product Role record.
- When a RIM document to be CrossLinked has a valid Site value (according to the Clinical Operations Site), the connection populates it accordingly. If the Site does not exist in Clinical Operations, the connection generates a User Exception Message.
- By default, when the Clinical source document does not include a Product value, Vault defaults Products on the RIM CrossLink based on the Product Family Clinical Study relationship for every Study referenced by the source document. Contact Veeva Support to enable an optional setting by which Vault instead defaults Products on the RIM CrossLink based on the Primary Product Family assigned to the Study referenced by the source document.
- When the RIM document to be CrossLinked has a “European Union” value in the Country field, Vault populates all relevant study countries (where the Country belongs to the “European Union” Jurisdiction) on the Clinical CrossLink.
- The Jurisdiction Country object must be configured in order to utilize this mapping logic. Contact Veeva Support for the recommended mappings.
- In order for Vault to populate a Clinical CrossLink’s Study Country field via the jurisdiction and country mapping logic, a RIM country value which is considered a Clinical Jurisdiction cannot be included in Clinical’s Country object. In the European Union example, this means the RIM Vault’s European Union country cannot be both a Country and a Jurisdiction in Clinical.
- If the RIM source document includes a Study that is archived in Clinical, then the Study is removed from the CrossLink before creation or update.
- All Products on the Clinical CrossLink document must be a Study Product for at least one Study on the document. If this is not true, then a user exception message is created and the CrossLink fails to create or update.
Additional Considerations
- When a new Study Product record is created in Clinical Operations, the connection does not automatically reprocess any CrossLinks to add additional Studies to documents.
- When users delete documents or object records in one Vault, Vault does not delete the associated documents or object records in the connected Vault.
About Record Creation & Update
When creating a record through the connection, Vault also populates the Link (link__sys) field on the target record with the source record’s Global ID (global_id__sys). These fields let Vault know which records to update in the target Vault when data is updated in the source Vault.
About Document Creation & Update
Vault creates new CrossLink documents in the steady state defined in the target Vault’s document lifecycle of a given document type. If the document’s type has multiple lifecycles, Vault applies the first lifecycle available. The connection creates CrossLink documents using the Specific Document Version binding rule. See details about source binding rules below.
The RIM-Clinical Operations Connection creates, up-versions and syncs metadata for documents in the target Vault that are configured to transfer between Vault applications:
- When a source document enters its Steady state, the Connection creates a CrossLink in the target Vault in the Steady state of the target’s Document Type defined on the Document Lifecycle. Vault also applies any entry actions configured on that lifecycle state, for example, setting the version number to a major version.
- If a Connection-created CrossLink exists on the target Vault and a new version of the source document is created and moved to a steady state, the Connection up-versions the Target CrossLink and creates a new CrossLink version.
- When the fields on a Steady state source document are modified in the source Vault, Vault updates those fields on the CrossLink document in the target Vault.
- If the connection has never run, Vault only transfers the latest Steady state version of the document.
- Document versions created in the target Vault are set by the target Vault and bound to the versions from which they were created in the source Vault.
- When enabled by Veeva, Vault transfers any Superseded versions of a document created since the last time the connection ran.
- If there are both Steady state and Superseded versions of the source document in the source Vault that have not been transferred to the target Vault since the last time the connection ran, Vault transfers all of these document versions.
- The Documents Integration is triggered when (1) a source document in-scope for transfer enters the Steady State and (2) a source document that has a Connection-created CrossLink has a metadata update. Updates on an Approved source document that does not have a Connection-created CrossLink does not trigger the Connection.
Note: Vault does not apply document reuse functionality to CrossLink documents in Clinical Operations Vaults that were transferred from RIM Vaults.
About Source Binding Rules
If a CrossLink document does not already exist in the target Vault, Vault creates the CrossLink document and binds it to the current document version in the source Vault. Vault will then update the CrossLink document only if a user in the source Vault specifically edits the version of the source document that is bound.
All CrossLink documents created via the RIM-Clinical Operations Connection have the Source Binding Rule set to Specific Document Version.